Multiplicity in oncology randomized trials: an approach to the KEYNOTE-B96 trial with a detour via bevacizumab
PD(L1) blockade in patients with ovarian cancer…
In ovarian cancer, despite numerous trials testing PD-(L)1 antibodies, results have been largely disappointing, leading to a broad consensus that these therapies show limited benefit. Below are quotes from recent publications summarizing the overall outcomes in this field:
“Immune checkpoint inhibitors have shown limited efficacy in ovarian cancer” (here)
“Despite (…) the results of trials testing immune-checkpoint inhibitors (ICIs) in these patients thus far have been disappointing.” (here)
… and now enter a positive trial: KEYNOTE-B96
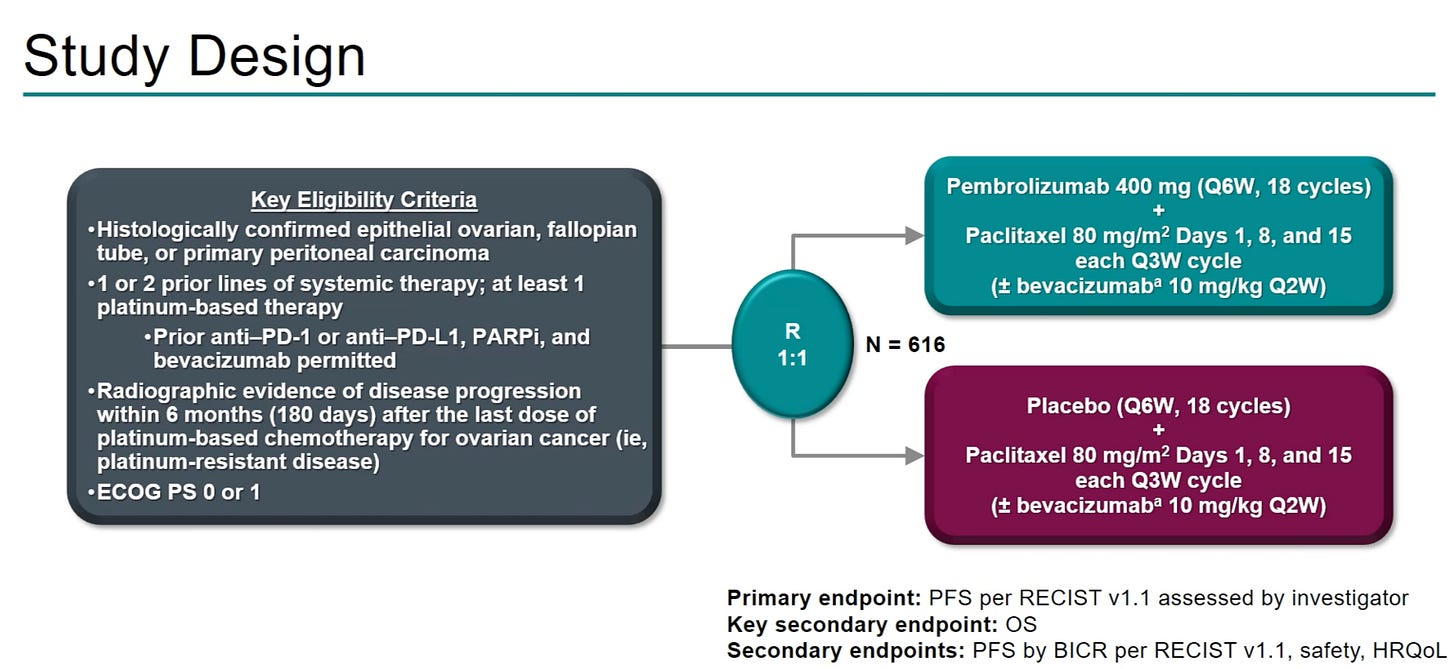
The ENGOT-ov65/KEYNOTE-B96 trial (NCT05116189) was a global, randomized, double-blind, phase III study evaluating pembrolizumab plus weekly paclitaxel with or without bevacizumab versus placebo plus the same regimen in patients with platinum-resistant recurrent ovarian cancer. The trial enrolled platinum-resistant patients with epithelial ovarian, fallopian tube, or primary peritoneal carcinoma (up to 2 prior lines of therapy including platinum). The primary endpoint was investigator assessed progression-free survival (PFS) in PD-L1 CPS ≥1 and all patients; overall survival (OS) was a secondary endpoint.
In a May 2025 press release, the trial was reported to demonstrate that adding pembrolizumab improved progression-free survival (PFS) across all patients and produced a statistically significant overall survival (OS) gain in PD-L1–positive patients compared with chemotherapy with or without bevacizumab. These results will first be presented during one of the Presidential Sessions at ESMO 2025.
Understanding multiplicity in oncology: a detour with bevacizumab
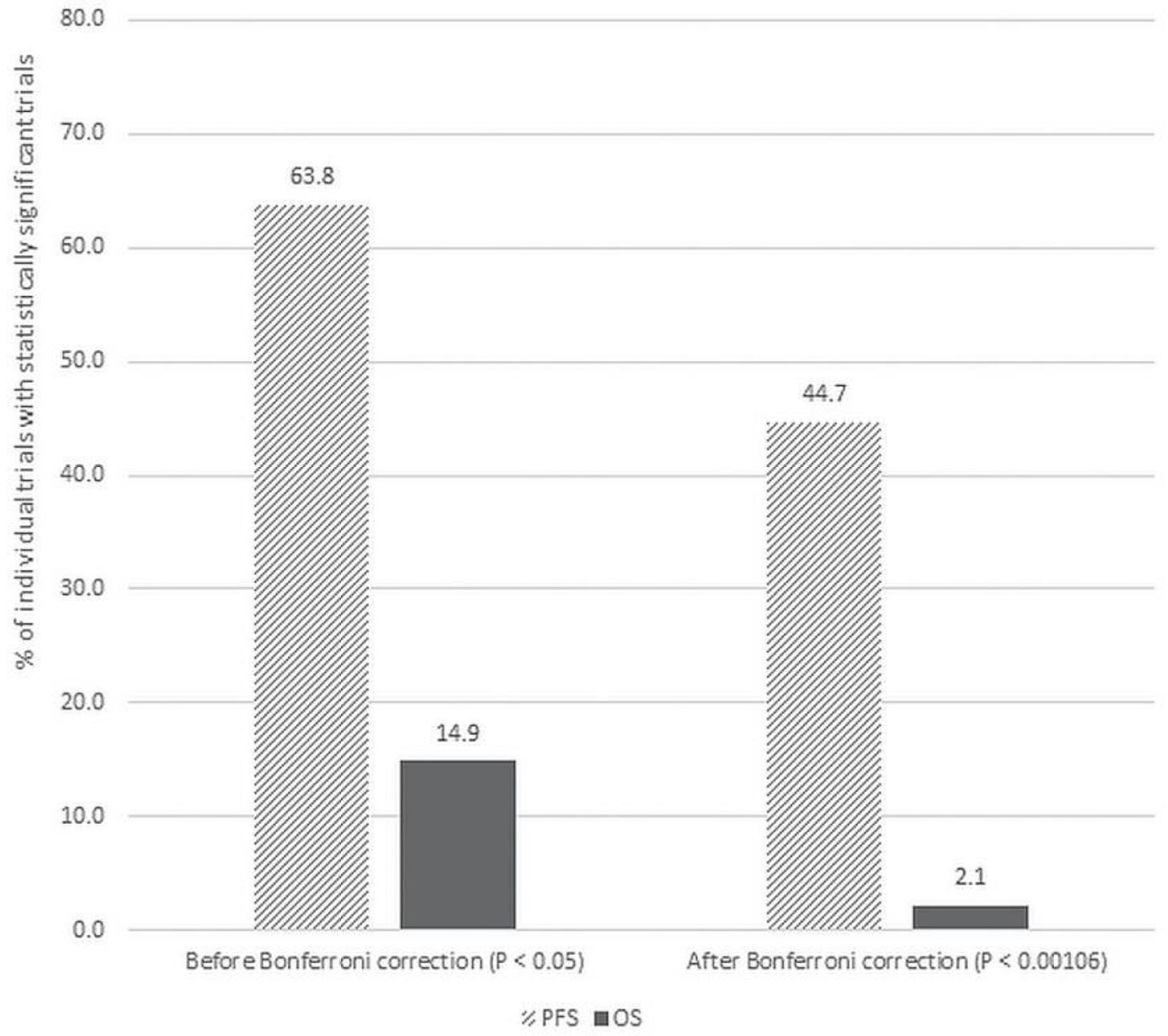
What is multiplicity, and why is it increasingly important to consider in the modern era of clinical trials? In 2019, Nathan Gay, Derrick Tao, and Vinay Prasad explained this concept and illustrated it using bevacizumab as an example. In their publication, they wrote:
“If a single drug is tested in many tumour types, the interpretation of any single trial result must account for the number of times that the agent is trialled. Drugs tested extensively might benefit from a portfolio-level analysis.”
In other words, when considering an entire portfolio of trials, the classical interpretation of the p-value may no longer hold, it may need to be adjusted using statistical methods such as the Bonferroni correction. Applying this approach to bevacizumab trials, the authors performed this analysis and concluded:
“Although 21 of 30 trials (70.0%) reporting a statistically significant PFS retained that claim after the Bonferroni correction, only 1 of 8 (12.5%) maintained its OS benefit.”
Takeaway point
With KEYNOTE-B96, we can apply the same portfolio-level perspective—not just across the anti–PD-(L)1 class of compounds, but even more meaningfully within a single tumor type, ovarian cancer.
In other words, while the results should not be dismissed outright, a single positive trial after numerous negative ones warrants particularly cautious interpretation.
I look forward to seeing the data presentation. We’ll likely have to wait for the full publication to examine the protocol and statistical plan in detail—were there any changes? For instance, the trial reportedly included 645 patients, whereas the 2022 ASCO abstract mentioned 616; was this difference due to a modification in the statistical plan or another amendment? As the trial was likely not powered for overall survival, careful interpretation of the survival results—including censoring patterns—will be essential.
We also know from the PARP inhibitor trials in ovarian cancer that a long follow-up is required to fully assess overall survival (OS) outcomes (see our paper here).
Today’s takeaway, however, is the importance of approaching any single trial through the lens of multiplicity in oncology.