Oncology “Me-Too” Drugs Compared With Original Drugs in Randomized Clinical Trials
This is the title of our recent JAMA Internal Medicine publication.
« Me-too » or « next-in-class » compounds are drugs with similar mechanisms of action. While some advantages, such as better efficacy or tolerance can be proposed, these advantages may remain theoretical without direct comparative evidence.
In this work, published in the September issue of JAMA Internal Medicine, our research question was simple: how often randomized clinical trials (RCTs) compare next-in-class and first-in-class drugs?
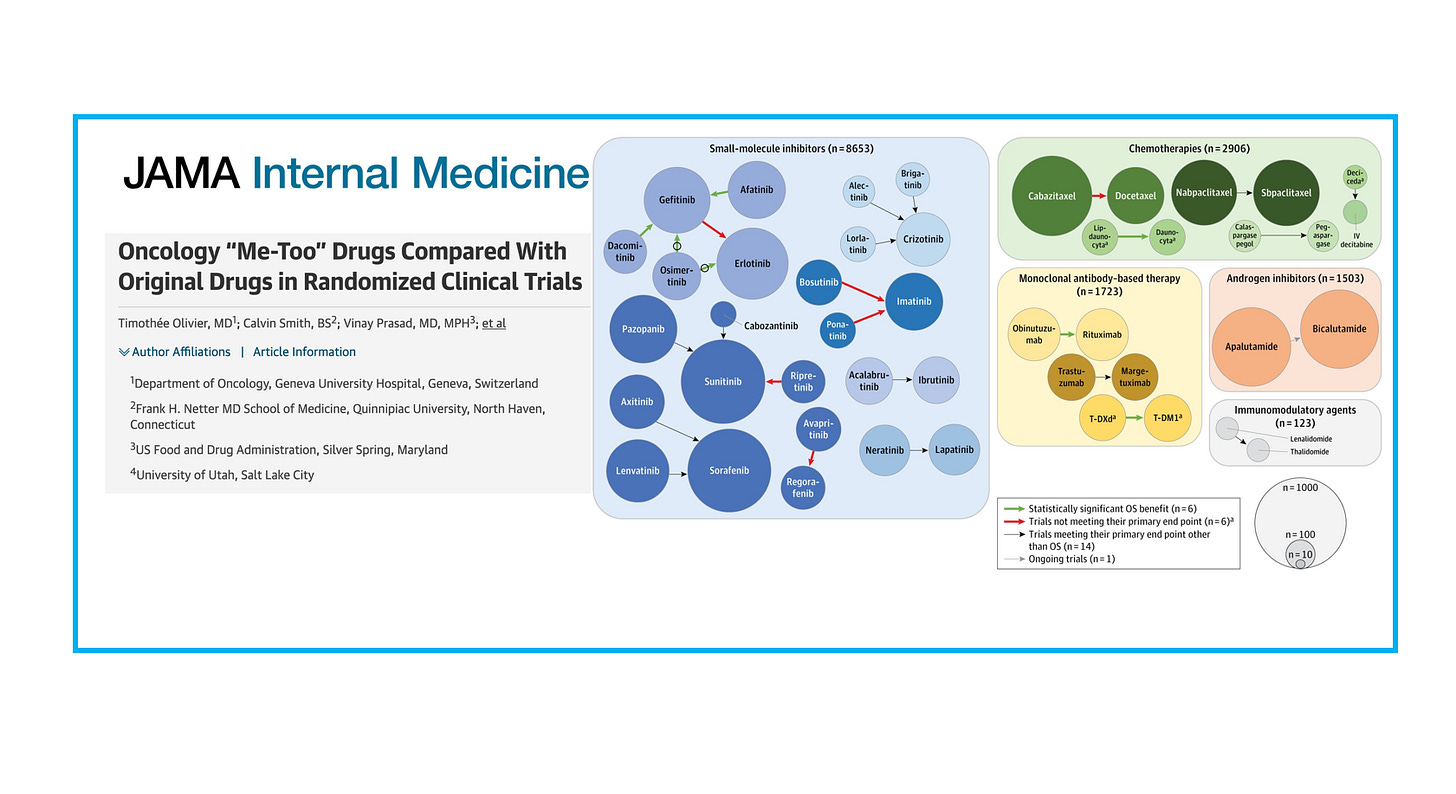
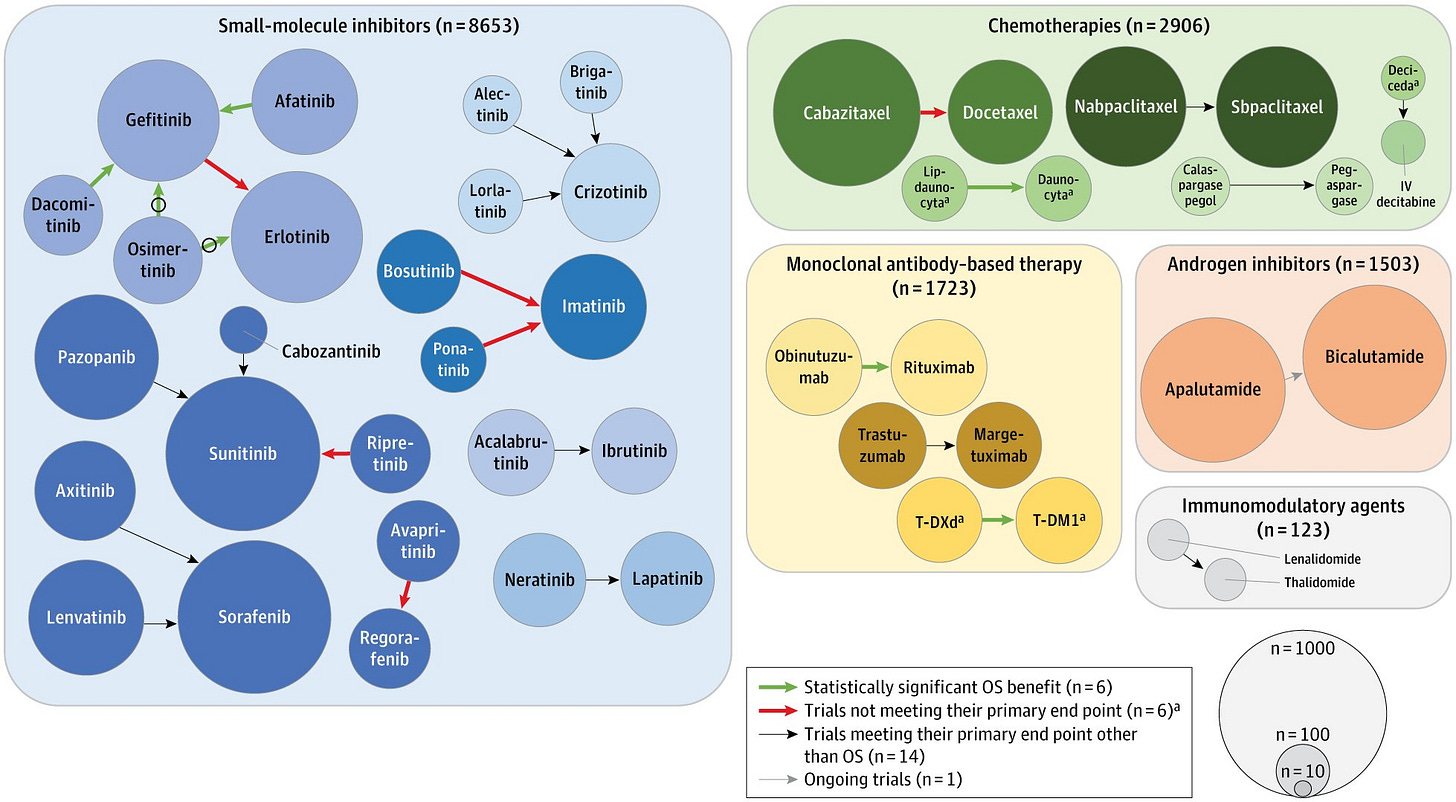
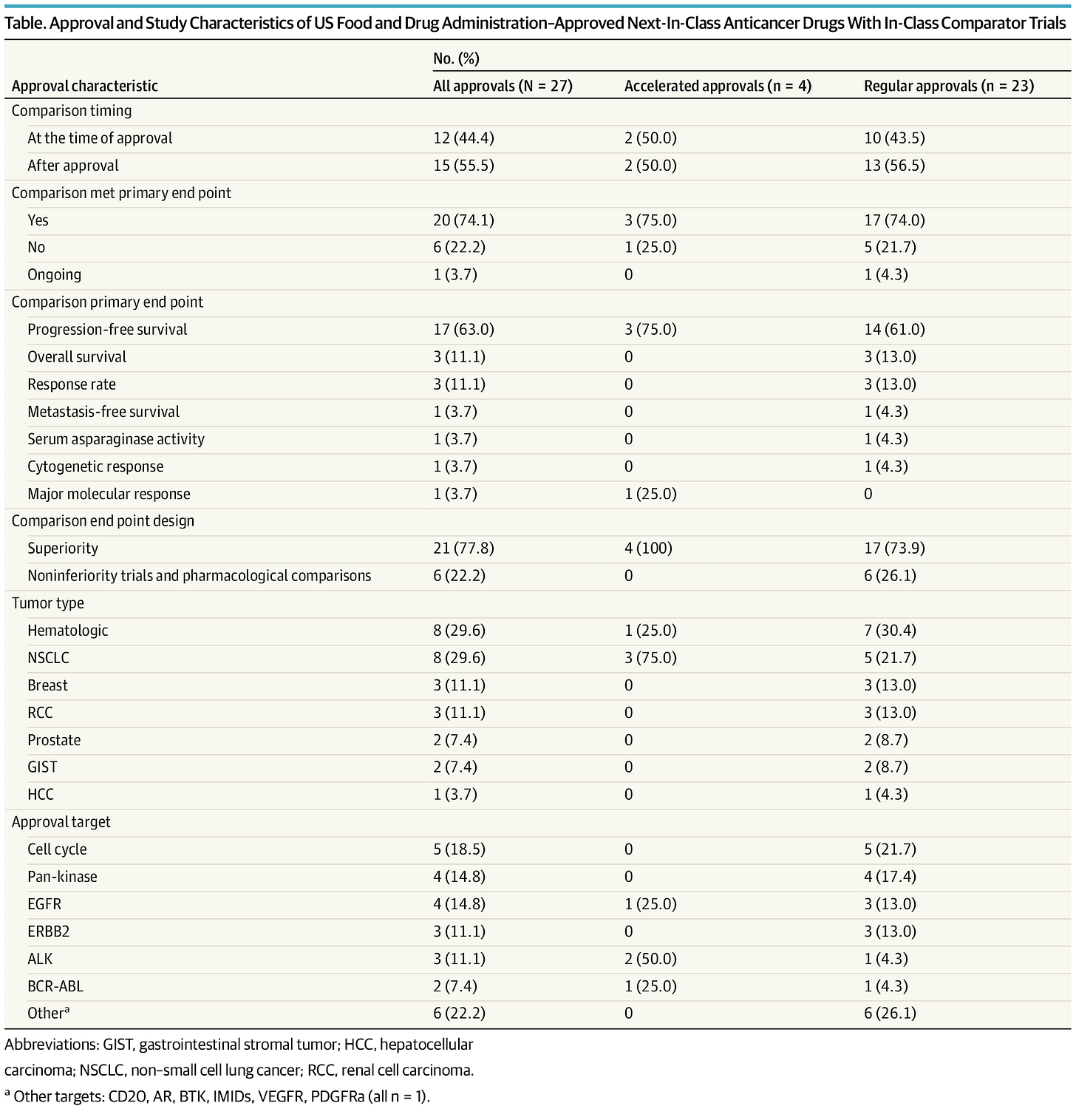
Out of 332 FDA approvals between 2009 and 2020, we included 94 approvals of a next-in-class compound. We found that 27 trials had a randomized trial with an in-class counterpart, but only 6 trials (6%) ultimately demonstrated a survival advantage.
Below is the key figure, with the size of circles representing the number of patients for a specific compound, and arrows representing head-to-head trials. The color of the arrows indicates whether the trial showed a survival gain (green, 6 trials), did not meet their primary endpoint (red, 6 trials!), or met their primary endpoint other than OS (14 trials).
However, in many instances, there were no head-to-head RCTs at all, making it difficult to assess the true therapeutic value of next-in-class drugs.
Our conclusion: “These results suggest a need for regulatory bodies to incentivize within-class RCTs. Such evidence would help determine whether next-in-class drugs provide meaningful advances in cancer care. This is particularly important given that me-too drugs have not led to price competition in oncology, and many are approved through single-group trials or studies using suboptimal controls. Although not always possible if drug development happened concurrently, future regulatory frameworks should consider mandating head-to-head trials against existing in-class agents whenever feasible.”
Check out our full article published in JAMA Internal Medicine for more granular results, including a breakdown by the type of approval (accelerated / regular) as seen in the table below, free link here!



Thank you great work
I would love your take on which classes of drugs you see as most promising to make a meaningful step in cancer treatment. ADC? Bispecifics? It seems like not much since keytruda