Solving Racial Inequalities in HLA-Restricted Therapies: Lessons from ESMO 2025.
Access to HLA-restricted therapy differs according to patients ethnicity, and should ideally be overcome.
Two studies presented at ESMO 2025 highlight the potential of HLA-restricted therapies, while at the same time confirming that such approaches are inherently unequal in terms of access depending on one’s race or ethnicity, as we previously showed with Vinay Prasad, Alyson Haslam and Jordan Tuia in a JAMA Network Oncology publication. In this post, I want to show what these two studies demonstrate in light of our work.
An entire drug pipeline is based on a patient’s race.
In our 2023 publication, we showed for the first time in the history of drug development that an entire drug pipeline is unequally accessible according to patient’s race or ethnicity.
The Major Histocompatibility Complex (MHC) helps the immune system distinguish between “self” and “non-self.” In humans, it’s the Human Leukocyte Antigen (HLA) complex — highly variable genes on chromosome 6 that give each person a unique immune profile. In medicine, the HLA system is crucial, as an example, for transplantations to reduce the risk of rejection by ensuring a good “match” between donor and recipient based on their HLA genes.
In our work, we examined all trials involving a therapeutic intervention that required participants to be positive for a specific HLA subtype(s), and identified 263 trials. Most of these trials focused on anti-cancer drugs (98%), and most were therapeutic vaccines (68%) or cellular therapies (28%).
Importantly, the HLA subtypes being used to recruit patient were the HLA-A2 or HLA-A*02:01 subtypes in 87% of trials.
We then estimated the likelihood of being eligible for such trials according to patients’ ethnicity. We estimated that African or African American individuals had the lowest likelihood of being enrolled in such trials (33%), when individuals of European or European Descent were 1.6 times more likely to be eligible (53%). Data for other ethnic groups are shown in Table 3.
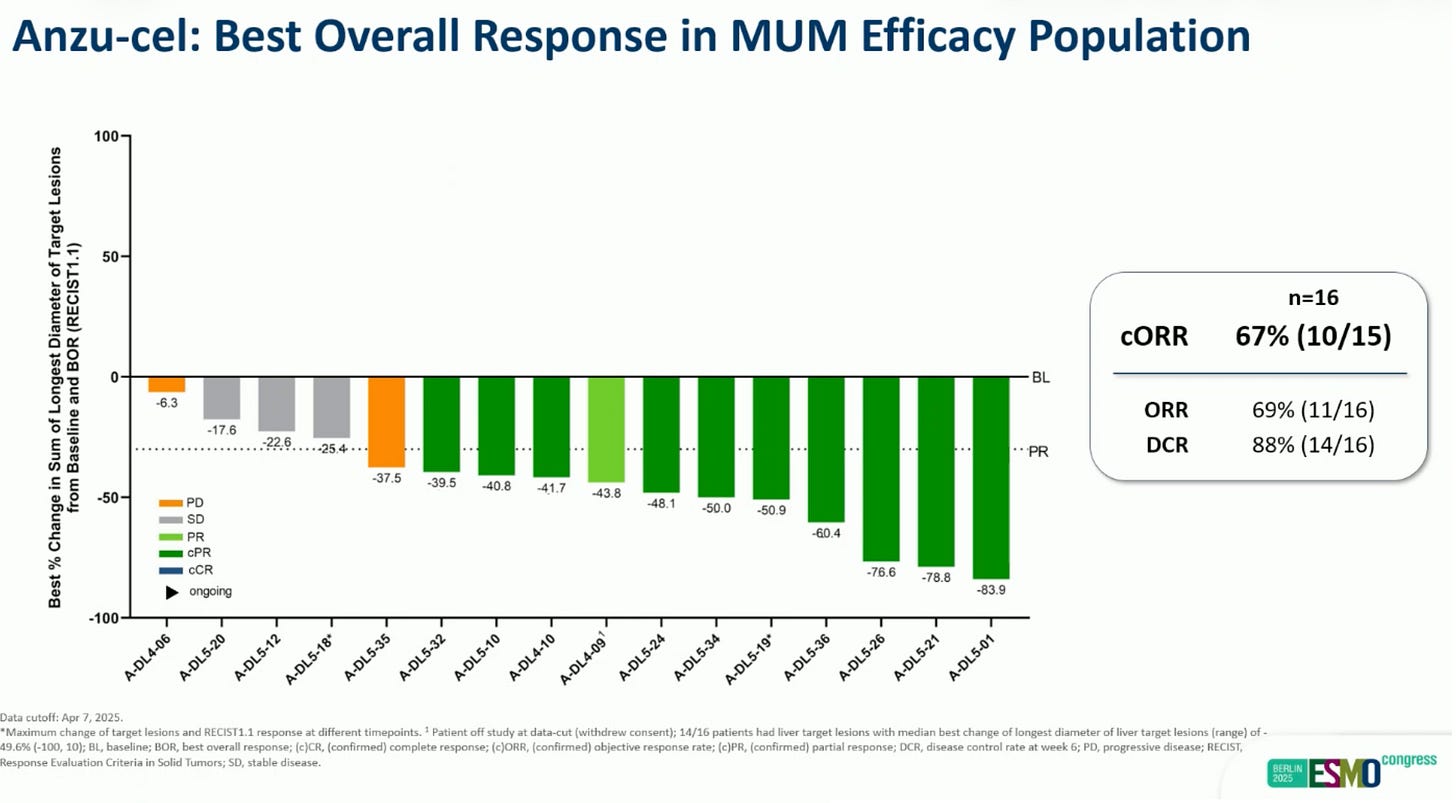
Anzu-cel in uveal melanoma: significant reponse rate, usual HLA-A*02:01 restriction.
A phase 1 trial in a rare cancer – uveal melanoma – made it to the Presidential session during ESMO 2025!
IMA203, or Anzu-Cel, is a T-cell-based therapy targeting PRAME, which is expressed in 90% of uveal melanoma. The study enrolled patients with recurrent or refractory solid tumours. The patients with uveal melanoma had a 67% confirmed overall response rate, with a 11-month median duration of response, which are quite impressive results in this disease. IMA203, or Anzu-cel, is a HLA-restricted therapy and only HLA-A*02:01-positive patients could receive the therapy. (abstract here)
A new HLA-A*11:01-restricted therapy has been developed in… China!
In an early trial of NW-301V, a T cell therapy targeting the KRAS G12V mutation, 8 patients with advanced colorectal or pancreatic cancer were treated. Three patients (37.5%) had partial response. (abstract here)
However, this novel HLA-restricted therapy now selects only patients with the HLA-A*11:01 immune type.
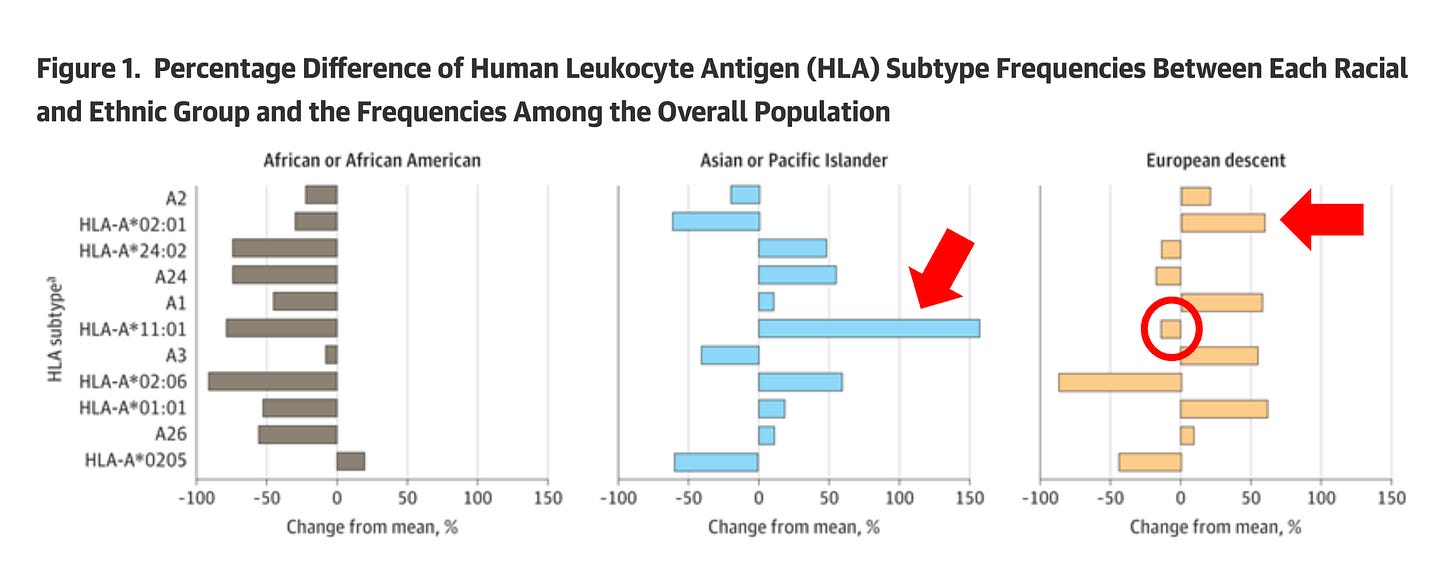
Why did the Chinese investigators choose HLA-A*11:01 instead of HLA A2:01?
The answer is straightforward when you examine Figure 1 from our publication (below). Compared to frequencies in the overall population, HLA-A*02:01 is more common in European descent (indicated by the red arrow on the right), while HLA-A*11:01 is the most prevalent in Asian or Pacific Islander populations (indicated by the red arrow in the middle) and is less common in European descent (indicated by the red circle).
In other words, the Chinese investigators chose the most prevalent HLA in their population. While this choice makes sense from their standpoint, it demonstrates two key points:
Therapies can be developed for HLA types other than HLA-A*02:01.
HLA restriction still leads to unequal access to therapies.
HLA-restriction inequalities must ultimately be overcome.
By definition, because HLA subtypes are unequally prevalent across individuals, any HLA-based therapeutics will inherently be unequally accessible according to one’s race.
While both ESMO studies show encouraging results, they also highlight the issue of HLA restriction in anti-cancer therapies. Overcoming HLA restrictions may be biologically challenging; however, solutions should be implemented whenever possible. The research community must strive to ensure equal access to innovative treatments, regardless of race or ethnicity.
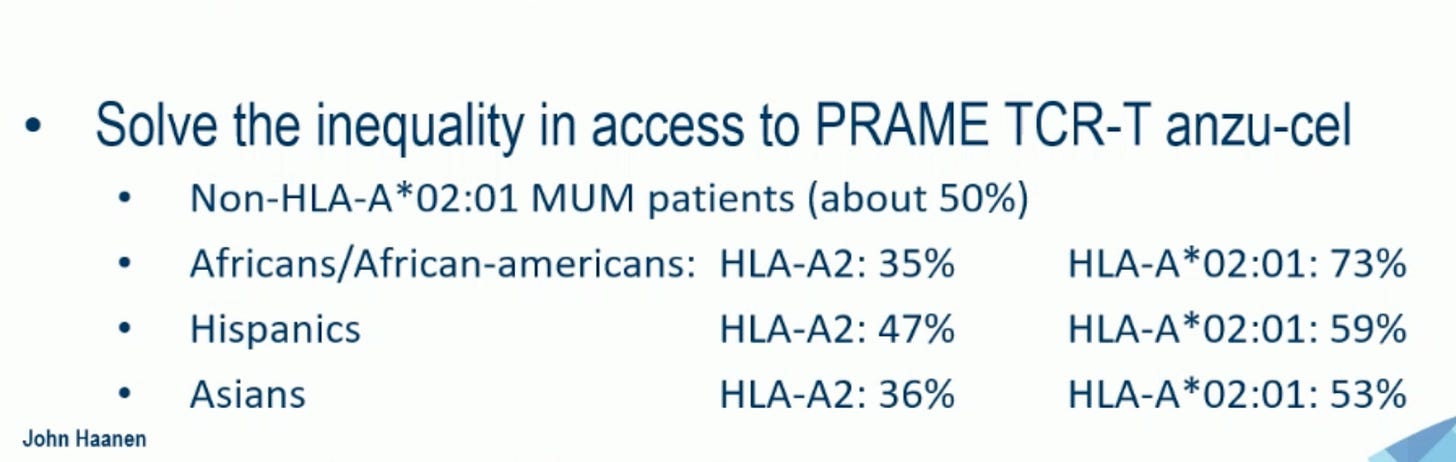
I would like to thank and congratulate my Colleague and Friend, Pr John Haanen, who discussed, during the ESMO 2025 presidential session, the IMA203 study in uveal melanoma and emphasized this point in his concluding remarks:
”Solve the inequality in access to PRAME TCR-T anzu-cel” (Pr John Haanen)






If cell therapies based on somatic cell nuclear transfer (SCNT), so called therapeutic cloning, were more ethically acceptable and practical, this would be less of an issue because the cells would be genetically matched to the intended patient. This is one of the advantages of personalized treatments over off-the shelf therapies.