Post-ESMO 2025 on Adjuvant CDK4/6 Inhibitors after MonarchE and NATALEE Updates.
With nearly 40% of patients newly diagnosed with breast cancer potentially eligible, the stakes are high.
As planned, the ESMO 2025 conference opened on Friday, October 17th, with updates on both trials: the first presentation of the survival benefit from MonarchE and a 5-year update from NATALEE.
MonarchE survival results
MonarchE enrolled patients with high-risk, HER2-negative, hormone receptor–positive early breast cancer and evaluated 2 years of adjuvant abemaciclib. The survival results, already announced by press release, were presented and published the same day in Annals of Oncology.
The most important result: a statistically significant overall survival benefit, with a 1.8-percentage-point difference after 7 years.

The survival benefit was expected, as it had been announced by press release. Most were awaiting the magnitude of the difference. For MonarchE, the OS benefit, translated into the Number Needed to Treat (NNT), is now 55.
In other words, according to the results, 55 patients need to be prescribed 2 years of abemaciclib to save one life after 7 years.
The main point is that I’m unsure whether such a survival benefit would be seen in practice, where patients have optimal access to CDK4/6 inhibitors for those who recur. Here is why.
Post-protocol, or subsequent therapy, is a key element to consider when a survival gain is reported. The main reason for suboptimal subsequent therapy is when trials are enrolling globally, including in countries where post-trial access to key drugs is limited or absent, thereby questioning the validity of the results in practices with optimal post-protocol care.
Here, as very good news, not only was subsequent therapy data reported, but it was also accompanied by a granular analysis. Here is the slide presented by Prof Stephen Johnston, MD, PhD.
The fact that access to CDK4/6 inhibitors was comparable in both groups for late recurrence led to the conclusion that this limited — but balanced — access was unlikely to explain the survival difference.
My interpretation differs, because this overlooks a key element: poor subsequent therapy access in both arms can artificially create or inflate a survival benefit, simply because the “dilution” of survival by later lines of therapy is reduced. Yet this “dilution” is precisely what we aim for in oncology — maximizing outcomes through optimal sequencing of treatments.
Such a phenomenon applies to any setting: when subsequent therapy is limited in both arms, any DFS or PFS benefit is more likely to translate into an OS benefit.
We illustrated this in one of our work published in BMC Cancer with Vinay Prasad and Alyson Haslam (Figure below).
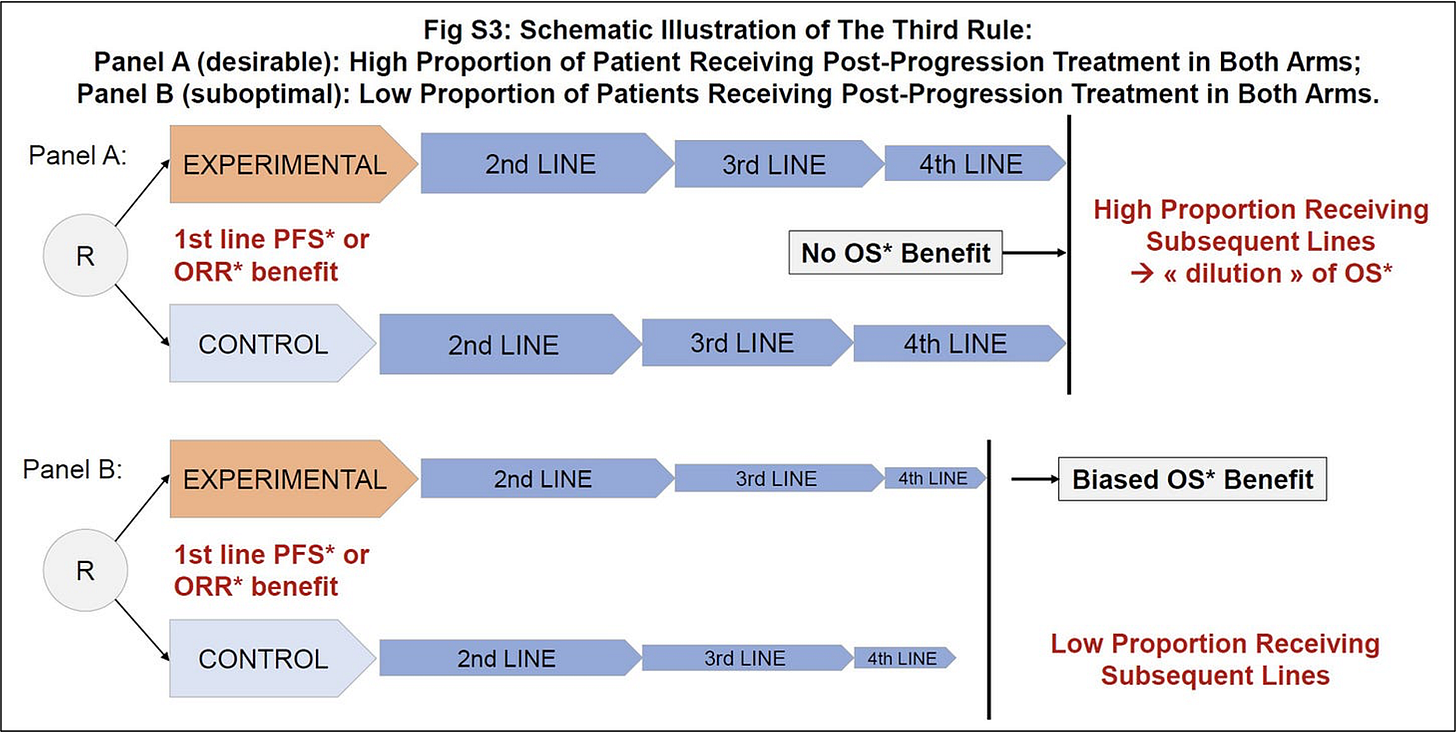
In MonarchE, among patients who received any first systemic treatment, only 38% in the experimental group and 57% in the control group received a CDK4/6 inhibitor as part of that therapy. Whether the survival benefit — already limited given the high NNT — would be maintained in settings with optimal access to subsequent therapy remains uncertain.
NATALEE 5-year updates
The NATALEE trial, with broader eligibility criteria, tested 3 years of adjuvant ribociclib. The 5-year updates were also presented and published the same day in ESMO Open.
As promised, the curves were digitized the same day, and a key takeaway is that the estimated number of early-censored patients remains overall stable — reinforcing our initial concerns that informative censoring likely occurred (more on that in a previous post, or in this publication).
Since the beginning, for both trials, the reliability of iDFS has been questioned due to the possibility of informative censoring. Even if one considers the iDFS benefit to be reliable, we should not forget that, by definition, in the adjuvant setting, a large proportion of patients will be treated with no benefit — an information best conveyed by the Number Needed to Treat (NNT).
Considering the 4.5–percentage point difference at 5 years for NATALEE, this would still mean that 22 patients would need to be treated to prevent one iDFS event.
Why This Topic Is So Important
It cannot be overstated how important these two trials are. Breast cancer has one of the highest incidences worldwide, and about 20% of patients could be eligible for MonarchE and 38% for NATALEE (with substantial overlap) (figure below from Swedish data).
Truly informed and shared decision-making.
This post is an appraisal of the data and is not intended to replace any medical decision. The adjuvant setting is the ideal context for truly informed and shared decision-making.
Whenever possible, the following points should be discussed with patients:
The definition of iDFS — it is not exclusively capturing “survival” events.
The limitations raised regarding the reliability of the iDFS benefit.
The toxicities associated with CDK4/6 inhibitors.
If mentioning the survival benefit observed in MonarchE, the potential limitation of suboptimal subsequent therapy should also be discussed.
The uncertainty of the benefit, even assuming results would replicate in our practice, particularly at the individual level (as illustrated by the number needed to treat for both iDFS and OS results).
Words matter:
Patients may or may not decide to undergo adjuvant therapy, and we should respect their decisions. In my opinion, we should reconsider some of the terms we were taught to use, such as “decline” or “refuse,” which may implicitly judge a patient’s choice. I personally prefer saying that the patient “decided”, which better reflects that a decision was made after discussion.
Avoid placing guilt:
Such discussions are also key to avoid placing additional burden or guilt on patients who cannot tolerate the therapy and have to stop it. I have encountered patients who were convinced that if they stopped adjuvant therapy, the cancer would automatically come back. Even if a recurrence occurs after a patient has stopped adjuvant therapy, no one can say that it happened because the therapy was discontinued.
This takes time, but that time is the essence of care — after all, it’s about the patient’s life.






In our country where the patient who will afford the treatment , I can’t consider this for them according to this results .
Hi Dr. Olivier - if only 38% of the experimental arm went on to receive CDK4/6 whereas 57% of the control arm did, wouldn’t that bias survival in benefit of the control arm?