Reduce Toxicity and Save Money: The Paper and Website to Bookmark for Alternative Dosing in Oncology!
The website to boomark: https://oncdoc.org/
A gem of a paper has just been published in the European Journal of Cancer. The article, titled “Potential Alternative Dosage Strategies for Patent-Protected Oral Cancer Drugs” is co-authored by Austin Wesevich, Liam J. Schmitt, and Mark J. Ratain.
Why should we consider alternate dosing in cancer treatment?
As the authors note upfront : “In the era of targeted small molecule drugs and immunotherapies to treat cancer, many oncology drugs have been approved at excessive dosages.” potentially leading to “unnecessary side effects for patients and potential financial waste for both payors and patients”
The topic is widely recognized as a priority in oncology, as reflected by the FDA’s launch of Project Optimus and by the growing attention it has received from regulators (free link here).
Here, using an interventional pharmacoeconomic approach, the authors examined oral oncology treatments to find where new dose-ranging trials are needed — and where current evidence already suggests that lower doses could safely be used.
They identified 90 patent-protected oral oncology drugs approved by the U.S. Food and Drug Administration between 2004 and 2024. Among these, more than half (50) had potential alternative dosing strategies. The key highlights are summarized below.
What is the level of evidence for alternate dosing strategies?
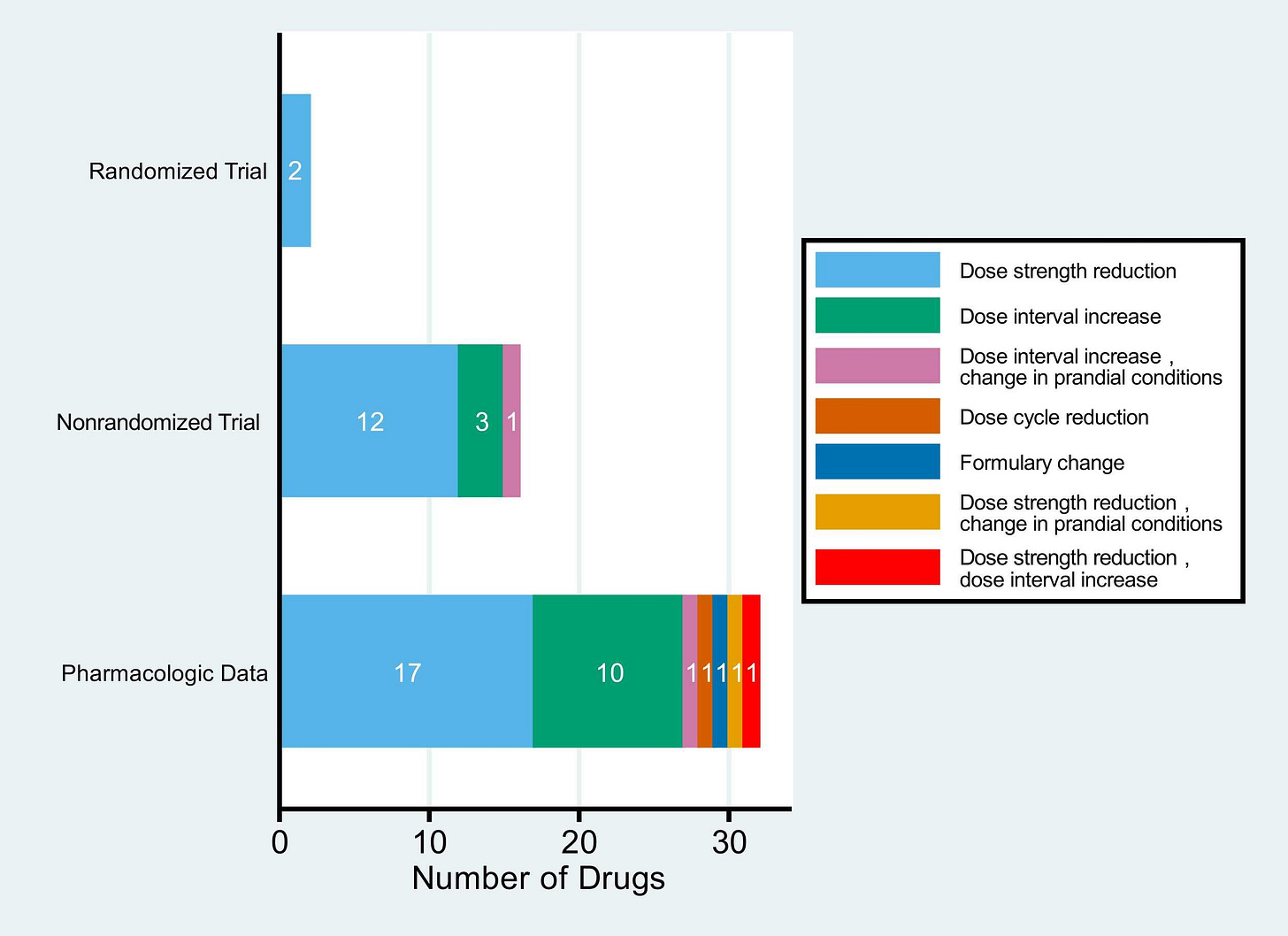
The authors detail their methods and references, as well as the level of evidence supporting their findings (see Figure 2 below). As shown, there are several ways to optimize dosing — such as reducing strength or increasing dosing intervals. The level of evidence is mostly based on non-randomized trials and pharmacological data, highlighting the need to incentivize both better dosing strategies upfront and high-level post-marketing studies.
Based on randomized trial data, the authors propose that “immediate dosage modification is warranted for sotorasib and ribociclib.”
The potential for annual savings of €20 billion
Even though the authors acknowledge some limitations in their methods, they estimated the potential for global annual savings if the alternative strategies they propose were applied — and concluded with the impressive figure of €20 billion per year.
They note : “The five drugs with the highest potential global reduction in annual sales revenue using alternative dosage strategies are enzalutamide (EUR 3.98 billion in savings), osimertinib (EUR 2.80 billion), ibrutinib (EUR 1.73 billion), olaparib (EUR 1.56 billion), and apalutamide (EUR 1.28 billion).”
Next steps: extend to off-patent generic oral drugs and parenteral compounds
Another reason to bookmark this website and closely follow these researchers is that they plan to expand their work to non-patented drugs as well as intravenous oncology drugs.
Congrats to the authors for this highly relevant work. This manuscript is truly impressive—the methods are clear, the writing is engaging, and the findings are significant. I highly recommend reading the full paper for deeper insights into the topic. (link here)





PD-1 inhibitors can be used at fractio of a dose. Low-dose immunotherapy has been shown to have efficacy in HNSCC and breast cancer.
Not a patent protected drug, but here’s an example. Despite the claim of similar side effect profiles, I suspect AE’s could be lower with alternate day dosing.
Double-blind, Randomized Trial of Alternative Letrozole Dosing
Regimens in Postmenopausal Women with Increased Breast
Cancer Risk
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4740217/pdf/nihms745043.pdf