Spotting informative censoring with longer follow-ups — the NADINA and NATALEE trials show two different patterns.
My new publication, “Identifying informative censoring from censoring patterns across successive follow-ups”, is out in the European Journal of Cancer and is openly accessible here.
Let me walk you through this work and explain why studying censoring patterns across longer follow-ups can help identify where informative censoring may have occurred.
The power of digitized curves: enabling estimates of censored patients
In short, when data sharing is absent or limited, or when the number of censored patients are not reported in Kaplan–Meier curves (after each number-at-risk), one can still use a powerful approach: first digitize the curves, then run code to generate pseudo–individual patient data (pseudo-IPD). These reconstructed data produce curves that closely mirror the originals and allow you to estimate the number of censored patients, among many other aspects worth exploring.
In this new work, I used digitization and the BREAKING-ICE App© to extract the number of censored patients at each time point across different follow-ups from the same trials.
In a trial with immature data, a high rate of early censoring is expected because many patients are still on-trial and are censored at the data cut-off — this is “administrative censoring.” With longer follow-up, these patients won’t remain early censored: they will either present the event or be censored later if they’re still on-trial without an event at the next data cut-off, and this continues with each subsequent update. Conversely, if early censoring does not decline as follow-up increases, the likelihood of informative censoring becomes higher.
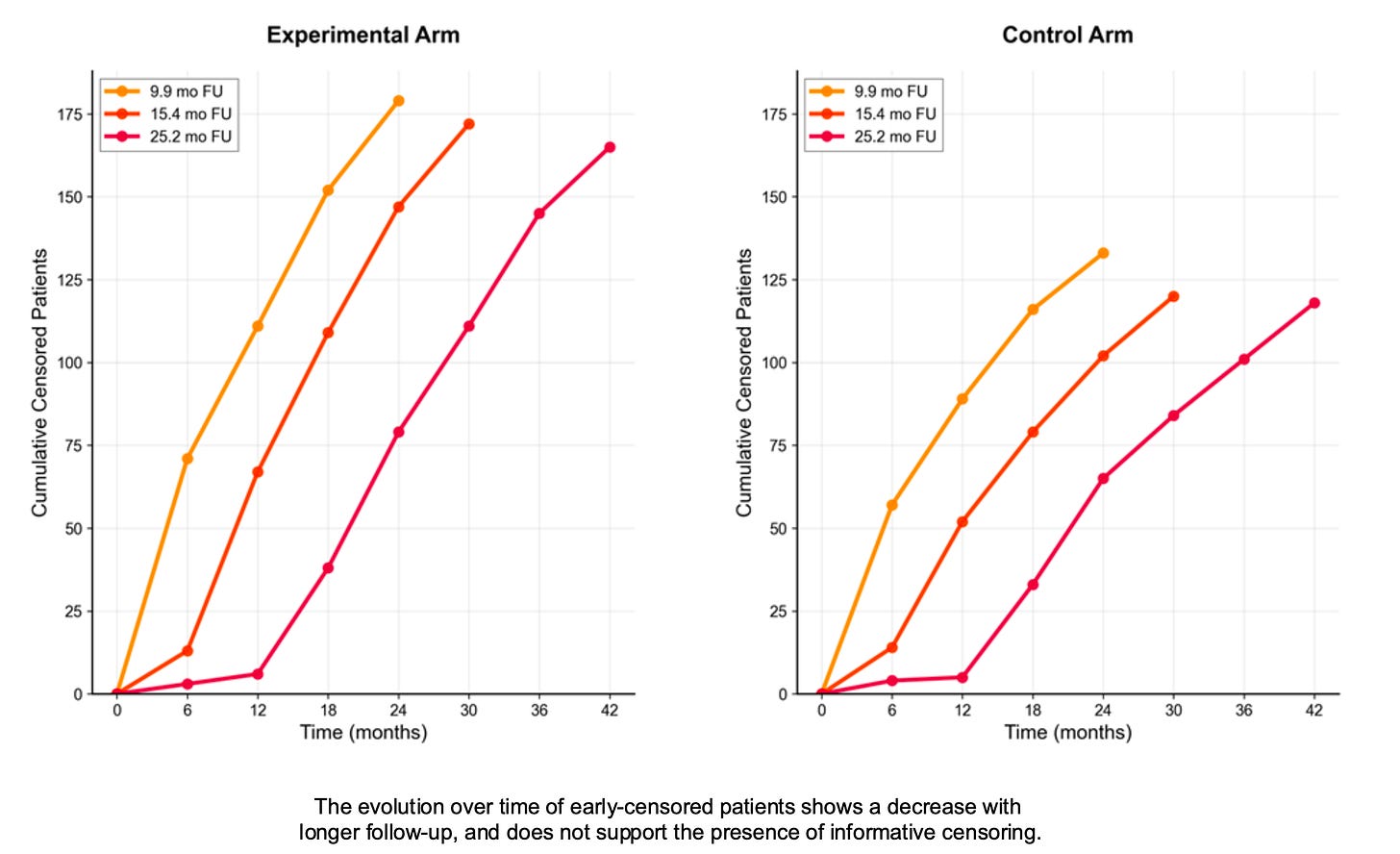
The NADINA trial: a reassuring evolution of the censoring pattern
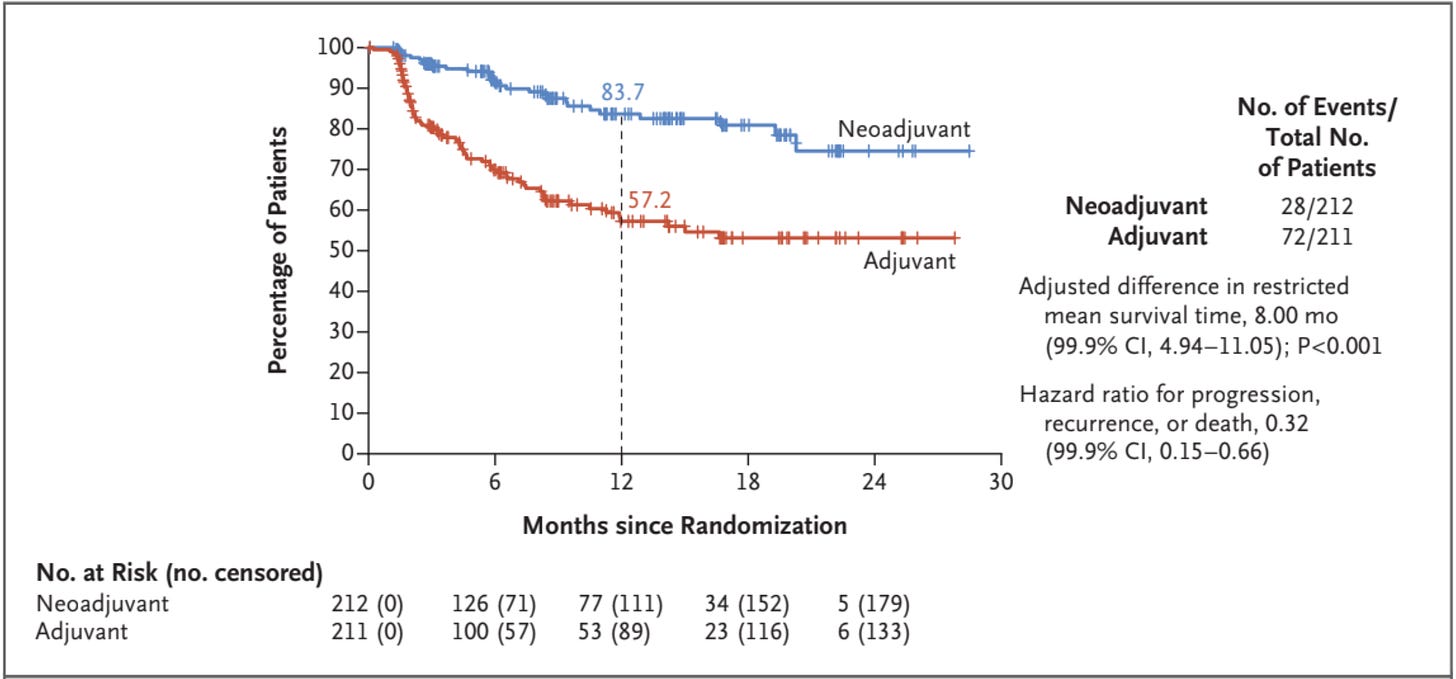
NADINA was a trial testing neoadjuvant immunotherapy (in one arm) compared to adjuvant therapy only, in patients with resectable, macroscopic stage III melanoma. When NADINA was first presented and published, there were questions about the possibility of informative censoring because of the high rates of censored patients — 33% and 27% in the experimental therapy and control groups, respectively. First, and this is commendable, the authors did report the number of censored patients in their publication (see below, numbers in bracket after the number at risk).
Moreover, they addressed the possibility of informative censoring by providing a reverse Kaplan–Meier plot in their supplementary appendix (more on this type of analysis here).
Based on updated data presented at ESMO 2024 and 2025, I was able to plot the censoring patterns with longer follow-ups. It clearly shows that the high early numbers decrease with longer follow-up, which is reassuring regarding informative censoring.
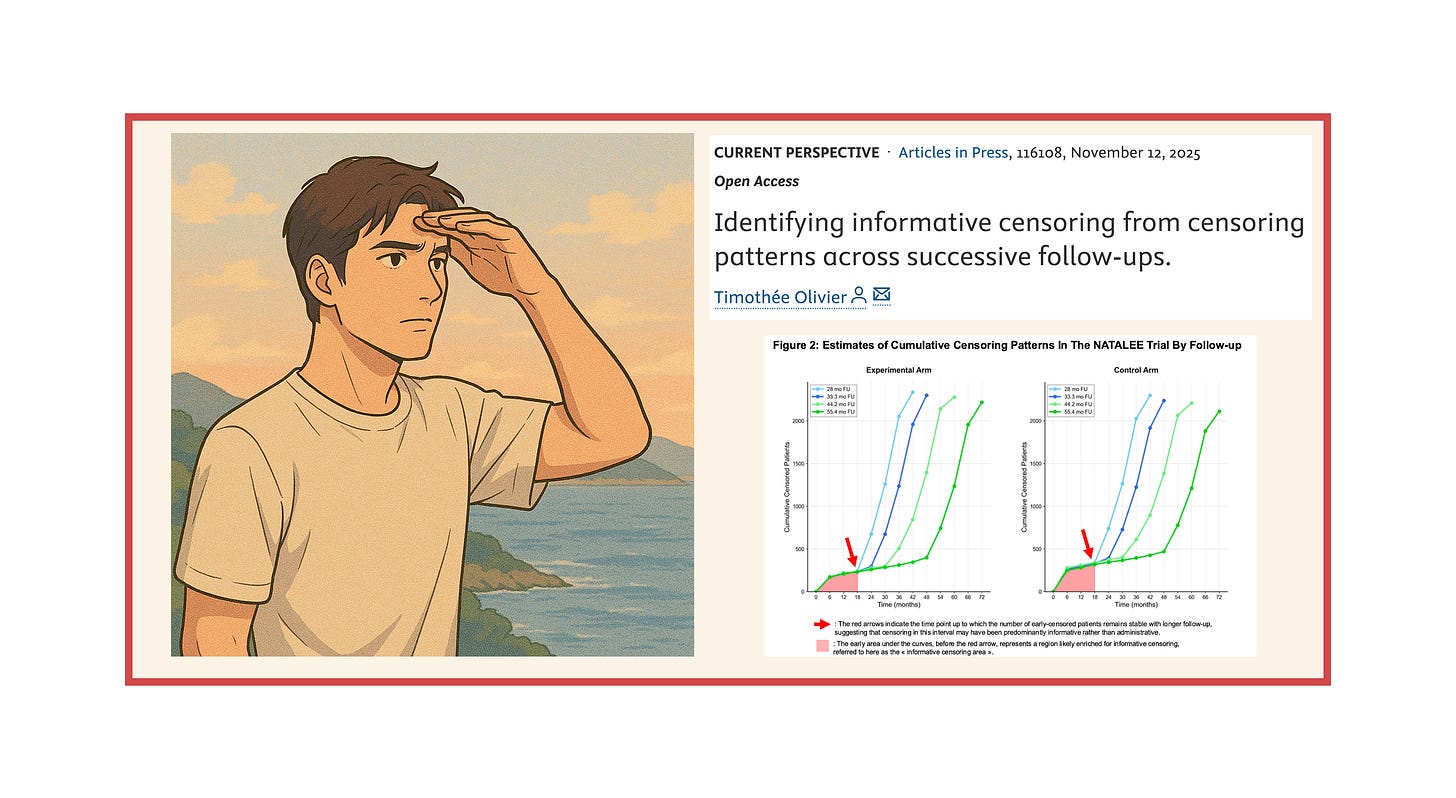
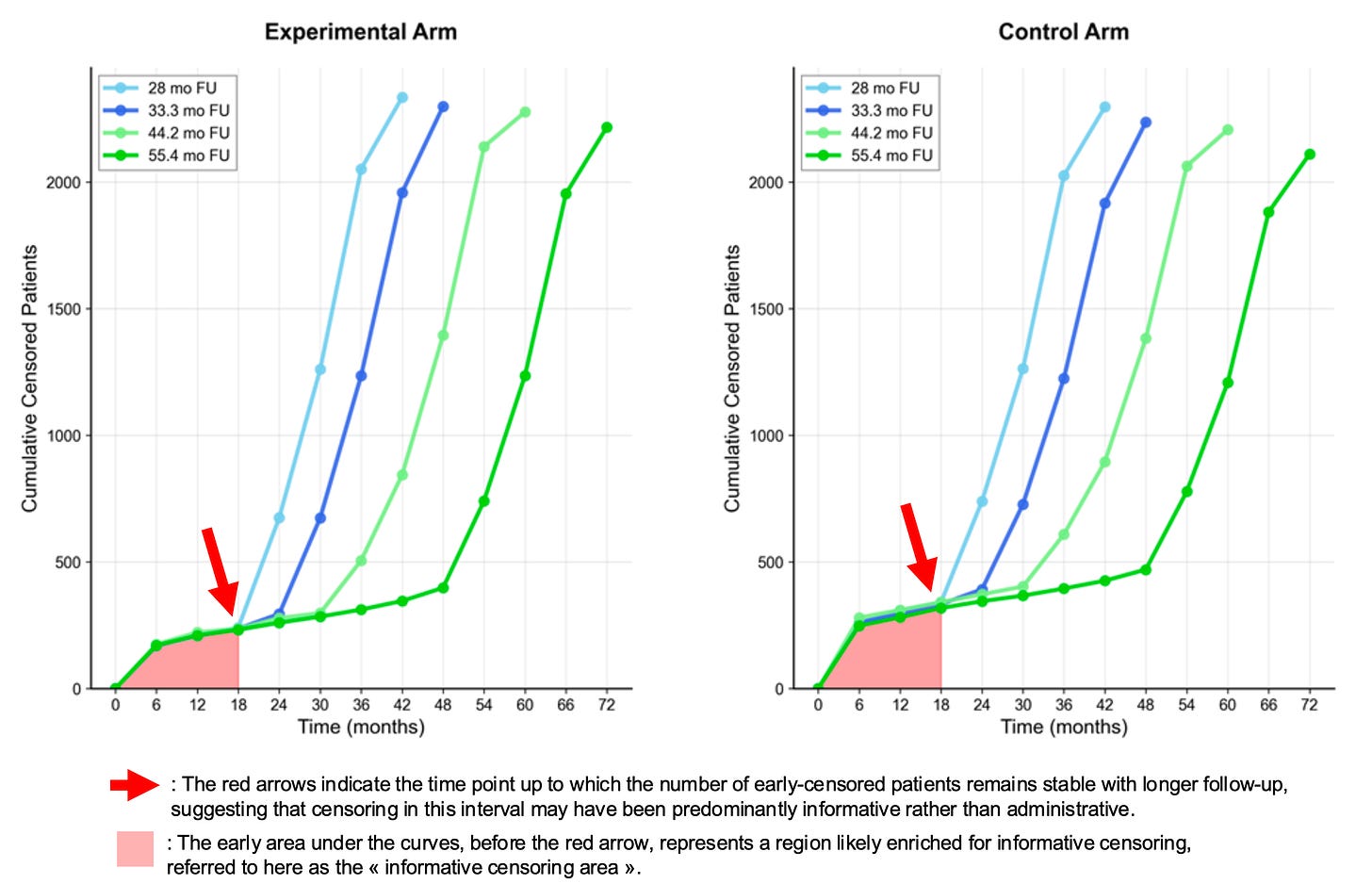
The NATALEE trial: a different pattern
NATALEE was a trial investigating the use of 3 years of adjuvant ribociclib in patients with early breast cancer. We and others have raised concerns about the possibility of informative censoring (also detailed here).
The censoring pattern is now evolving very differently with longer follow-ups. There is an area where high and early censoring remains stable, allowing us to identify an “informative censoring area” — here up to the first 18 months — where informative censoring likely occurred.
Of course, this does not mean it couldn’t occur later; however, the identification of an “informative censoring area” in NATALEE reasonably justifies conducting sensitivity analyses within the period where informative censoring most likely occured. This relevant window extends beyond 6 months—a period chosen in a recent publication—and restricting analyses to such an early interval may miss a substantial part where informative censoring could have occurred.
Significant early censoring? Wait for longer follow-up.
The main takeaway point is that when facing significant early censoring, longer follow-up may help distinguish administrative censoring from informative censoring. Regarding informative censoring, the more mature data in NADINA are reassuring, but this is not the case in NATALEE. Another way to address this issue—even with earlier follow-up—would be real data-sharing, which is often limited or absent.
“(…) when assessing trial outcomes, health technology assessment bodies or regulatory agencies may request longer follow-up to better determine the extent to which an early censoring pattern is likely to be informative.”
You can read the full paper here — it’s open access!



Great stuff! Have a question though. If the rate of early informative censoring is significant but equal between the arms, while the REASON for censoring is different (early dropout for toxicity in experimental and "disappointment" in control arm) would your method of the "censoring area" have a particular pattern?